Abstract
Background: Patients with hematologic malignancies relapsing after allogeneic blood or marrow transplantation (BMT) have limited response to conventional salvage therapies with an expected 1-year overall survival (OS) of <20%. Current strategies to treat relapsed hematopoietic cancer after BMT, such as chemotherapy and donor lymphocyte infusions (DLI) to enhance the immunologic graft-versus-leukemia (GVL) effect, have low efficacy and are associated with graft-versus-host disease (GVHD). An alternative strategy to boost the GVL effect is to infuse donor-derived T-cells targeting tumor-associated antigen (TAA) peptides as circulating TAA-specific T-cells are associated with maintenance of remission post-BMT. In this phase-I study, we evaluated the safety and clinical outcomes following administration of a novel T-cell therapeutic targeting three TAAs, WT1, PRAME and survivin in patients with acute leukemia who relapsed or were at high-risk of relapse after allogeneic BMT.
Methods: Lymphocytes obtained from the BMT donor were manufactured to target TAAs; WT1, PRAME and survivin, which are over-expressed and immunogenic in most hematologic malignancies. Patients received TAA-T infusions at doses of 0.5-4x10 7/m 2. Patients were evaluated for acute infusion-related toxicity, cytokine release syndrome (CRS), neurotoxicity, GVHD and monitored for clinical response post-infusion. T-cell receptor sequencing was conducted on the TAA-T product and the recipients' peripheral blood prior to and post-infusion to monitor persistence and expansion of the product.
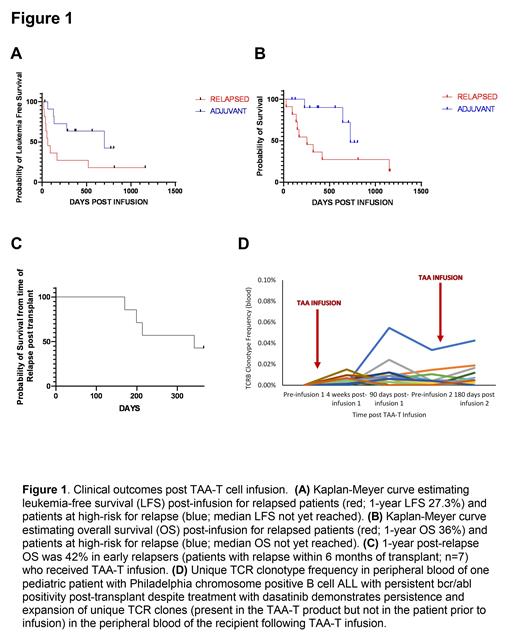
Results: Twenty-three BMT recipients with relapsed/refractory (n=11) and/or high-risk (n=12) acute myeloid leukemia (n=20) and acute lymphoblastic leukemia (n=3) were infused post-transplant. No patient developed CRS or neurotoxicity, and only one patient developed grade III GVHD. Of the patients who relapsed post-BMT and received bridging therapy, the majority (n=9/11) achieved complete hematologic remission before receiving TAA-T. Relapsed patients exhibited a 1-year OS of 36% and 1-year leukemia-free survival of 27.3% post-TAA-T (Figure 1A and B). The poorest prognosis patients (relapsed less than 6 months after transplant) exhibited a 1-year OS of 42.8% post-relapse (n=7) (Figure 1C). Median survival was not reached for high-risk patients who received pre-emptive TAA-T post-transplant (n=12) with eight of the nine (88.9%) evaluable patients at 1-year post-infusion remaining alive (Figure 1B). Of those, five (62.5%) were alive and in continued remission at the time of submission. Evaluation of unique T-cell receptor clonotypes (present in the product but not the recipient prior to infusion) demonstrated expansion and persistence in patients up to 1-year post-infusion (Figure 1D).
Conclusions: Although as a Phase-I study, concomitant anti-leukemic therapy was allowed, TAA-T cell therapy was shown to be safe and well-tolerated with sustained remissions observed in high-risk and relapsed patients. Moreover, adoptively transferred TAA-T expanded in vivo as detected by T-cell receptor V-beta (TCRVb) sequencing persisted up to at least 1-year post-infusion (Figure 1D). (ClinicalTrials.gov numbers, NCT002203902)
Cruz: Mana Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Catamaran Bio: Consultancy, Patents & Royalties, Research Funding. Hanley: Mana Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellevolve: Consultancy; Maxcyte: Membership on an entity's Board of Directors or advisory committees. Bollard: SOBI: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cabaletta Bio: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Catamaran Bio: Membership on an entity's Board of Directors or advisory committees; Cellectis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ROCHE: Consultancy, Honoraria; Repertoire Immune Medicines: Current equity holder in publicly-traded company; Neximmune: Current equity holder in publicly-traded company; Mana Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal